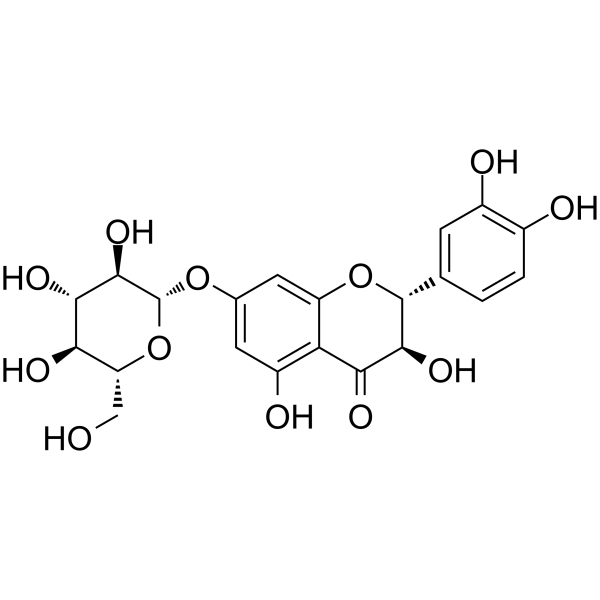
Taxifolin 7-O-β-D-glucoside
CAS No. 14292-40-1
Taxifolin 7-O-β-D-glucoside( Taxifolin 7-O-glucoside | Taxifolin 7-O-β-D-glucoside | Taxifolin 7-Glucoside )
Catalog No. M29433 CAS No. 14292-40-1
Taxifolin 7-O-β-D-glucoside is a flavonoid mainly found in endosperm and involved in defense against pathogens and UV damage.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 357 | Get Quote |


|
| 10MG | 529 | Get Quote |


|
| 25MG | 845 | Get Quote |


|
| 50MG | 1134 | Get Quote |


|
| 100MG | Get Quote | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameTaxifolin 7-O-β-D-glucoside
-
NoteResearch use only, not for human use.
-
Brief DescriptionTaxifolin 7-O-β-D-glucoside is a flavonoid mainly found in endosperm and involved in defense against pathogens and UV damage.
-
DescriptionTaxifolin 7-O-β-D-glucoside is a flavonoid mainly found in endosperm and involved in defense against pathogens and UV damage.
-
In Vitro——
-
In Vivo——
-
SynonymsTaxifolin 7-O-glucoside | Taxifolin 7-O-β-D-glucoside | Taxifolin 7-Glucoside
-
PathwayOthers
-
TargetOther Targets
-
Recptor——
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number14292-40-1
-
Formula Weight466.395
-
Molecular FormulaC21H22O12
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (214.41 mM)
-
SMILESOC[C@H]1O[C@@H](Oc2cc(O)c3C(=O)[C@H](O)[C@H](Oc3c2)c2ccc(O)c(O)c2)[C@H](O)[C@@H](O)[C@@H]1O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
SCP1-IN-1
SCP1-IN-1 (compound SH T-62) is a potent and selective covalent inhibitor of SCP1, promoting REST degradation and reducing transcriptional activity.
-
Emoxipine
Emoxipine is an antidepressant agent.The influence of emoxipine (2-ethyl-6-methyl-3-hydroxypyridine hydrochloride) and mexidol (2-ethyl-6-methyl-3-hydroxypyridine succinate) on the content of lipid peroxidation products in peripheral blood and the dynamics of clinical symptoms of gastrointestinal tract pathology has been studied in patients of middle and senile age with atherosclerosis in the abdominal aorta.
-
Methyl obacunoate
The fruits of Citrus reticulata Blanco.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


